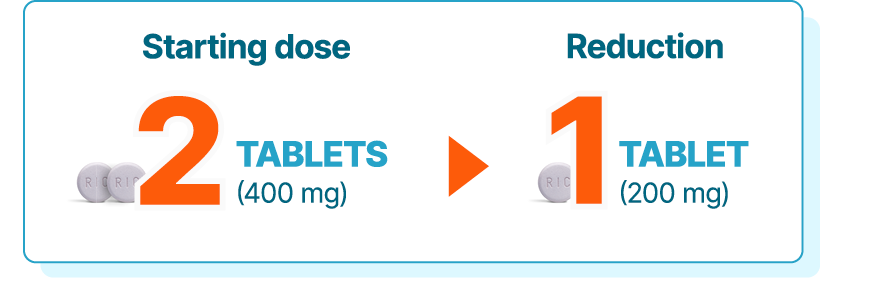
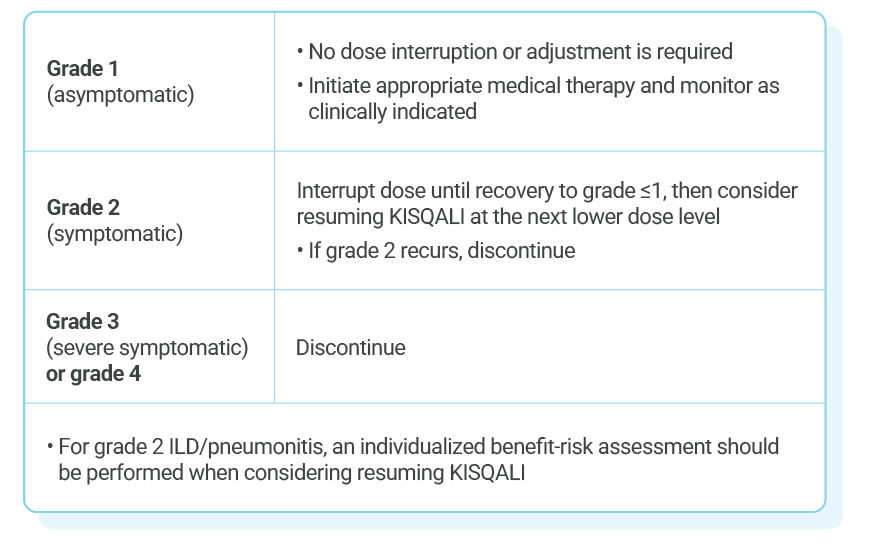
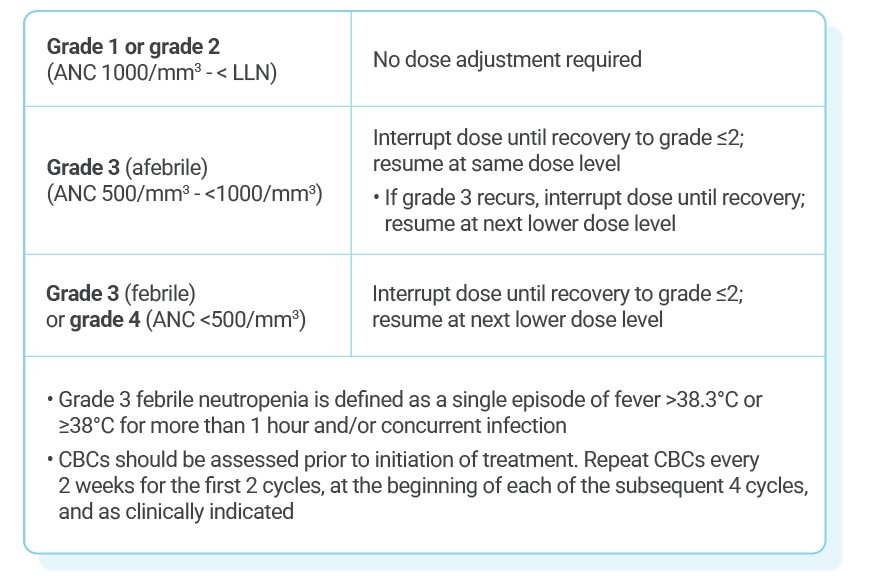
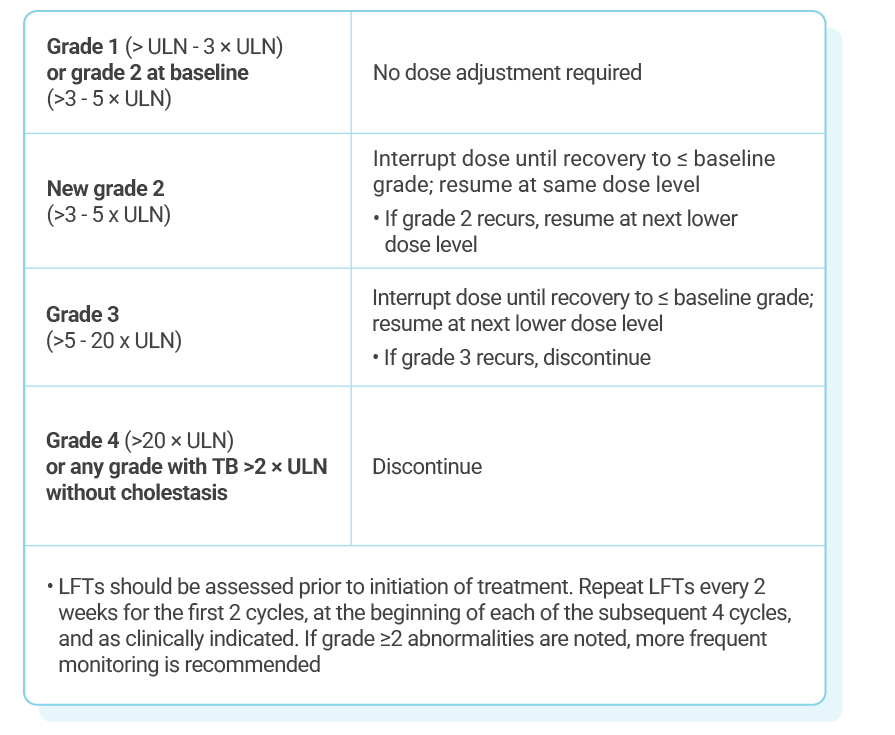
KISQALI single-strength tablets make dose reduction simple and convenient
Dose reductions with KISQALI mean no need for new mid-cycle prescriptions or additional costs
For patients with stage II/III HR+/HER2- eBC,
KISQALI is given as 400 mg (2 x 200-mg tablets) orally, once daily (3 weeks on, 1 week off) for 36 months with an AI1
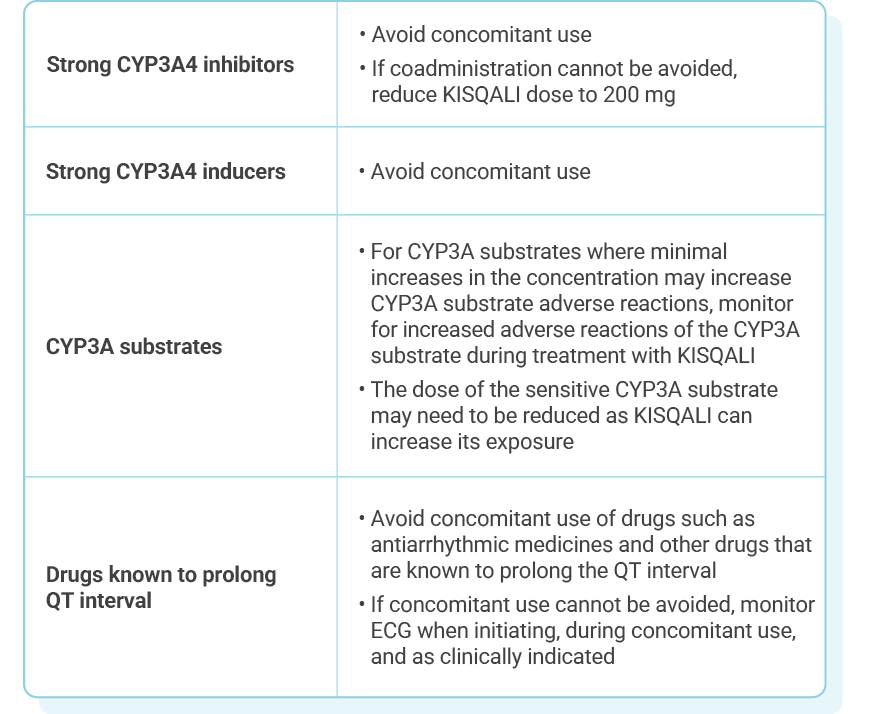
Review the full Prescribing Information for recommended dosing of selected AI
An LHRH agonist should be used concomitantly with AI in men and premenopausal women
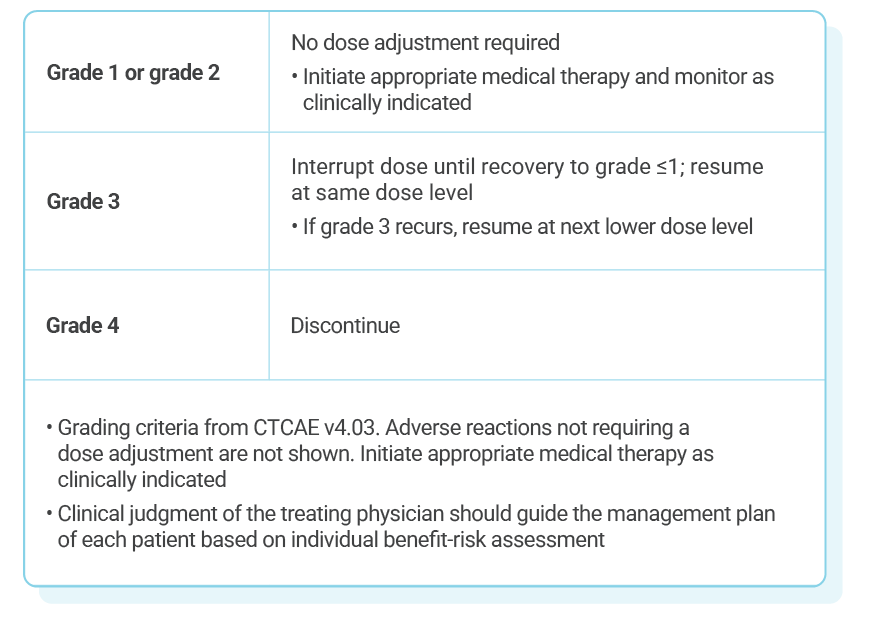
Dose adjustments for adverse reactions should be made by reducing the number of tablets taken1
If dose reduction below 200 mg/day is required, discontinue treatment
KISQALI dose modification is recommended based on individual safety and tolerability
KISQALI can be taken with or without food