Patient-reported health-related quality of life with KISQALI + AI vs AI alone in stage II/III HR+/HER2- eBC at high risk of recurrence
In NATALEE, physical functioning from the EORTC QLQ-C30 was the prespecified primary HRQOL outcome of interest1,2
AI, aromatase inhibitor; eBC, early breast cancer; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; HRQOL, health-related quality of life.
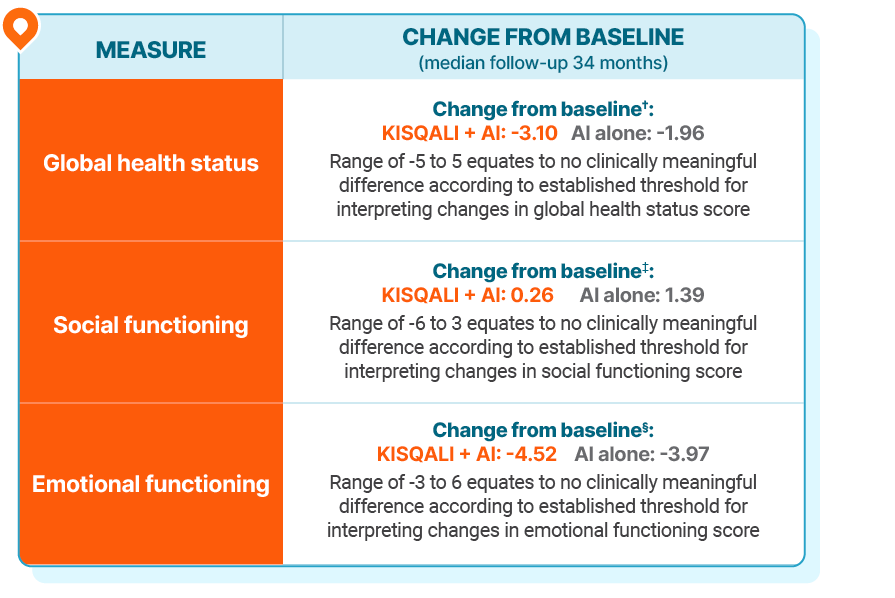
Additional HRQOL outcomes from the EORTC QLQ-C30 in NATALEE1,2
*Standard deviation from baseline values was 14.87 for KISQALI + AI treatment arm and 14.87 for AI alone; all changes were within 0.5 SD of baseline values.
†Standard deviation from baseline values was 17.67 for KISQALI + AI treatment arm and 17.77 for AI alone; all changes were within 0.5 SD of baseline values.
‡Standard deviation from baseline values was 22.55 for KISQALI + AI treatment arm and 22.36 for AI alone; all changes were within 0.5 SD of baseline values.
§Standard deviation from baseline values was 20.07 for KISQALI + AI treatment arm and 19.51 for AI alone; all changes were within 0.5 SD of baseline values.
HRQOL was a secondary end point measured by patient-reported outcomes and was assessed at baseline, every 12 weeks for the first 24 months of treatment and every 24 weeks after that, at end of treatment, at confirmation of first recurrence, and every 12 or 24 weeks after confirmation of distant recurrence1
There was no prespecified statistical procedure controlling for type 1 error
The HRQOL measures used in the NATALEE trial are not all inclusive and do not include assessment of all disease- or treatment-related symptoms
NATALEE was a randomized, multicenter, open-label, phase III study of KISQALI + letrozole or anastrozole (n=2549) vs letrozole or anastrozole (n=2552) for the adjuvant treatment of men and women with stage II/III HR+/HER2- eBC, including all those with node-positive or high-risk node negative disease (eligible stages and nodal status include: anatomic stage group IIB-III, or anatomic stage group IIA that is either node positive, or node negative with histologic grade 3, or histologic grade 2 with Ki-67 ≥20% and/or high risk by gene signature testing). HRQOL was a secondary end point; iDFS was the primary end point.1,3,4
iDFS, invasive disease-free survival; SD, standard deviation.