Help your patients with aggressive disease live more of the lives they love
POOLED ANALYSIS
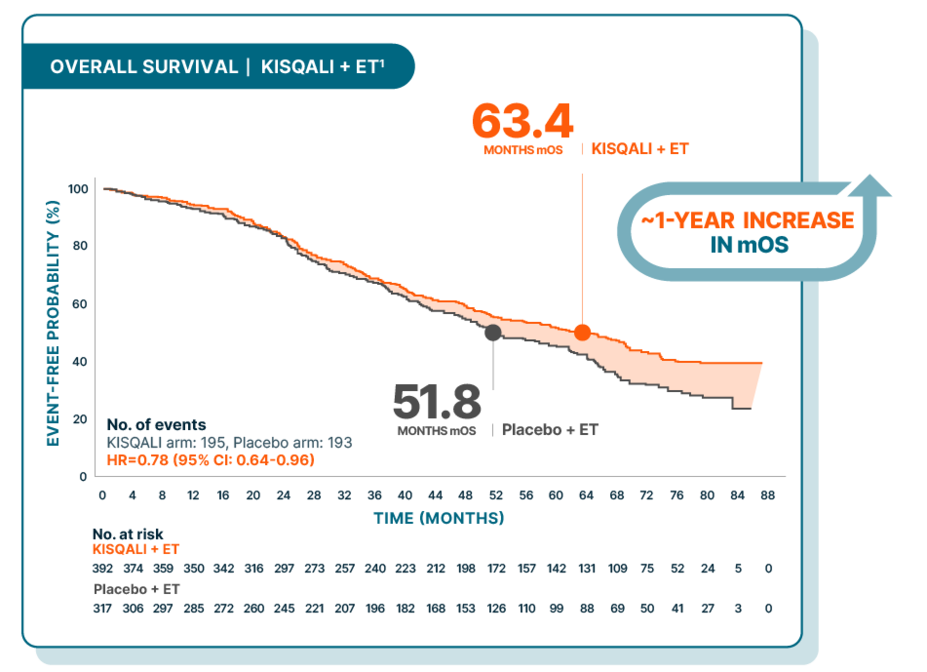
Over 5 years median overall survival in 1L patients with visceral disease across 3 phase III MONALEESA trials
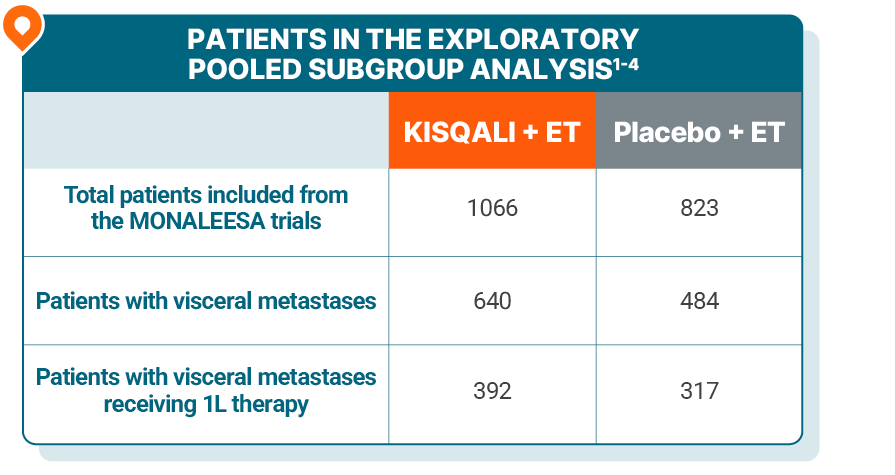
MONALEESA-2, -3, and -7: Exploratory, pooled subgroup analysis of 1L pre- and postmenopausal patients with visceral metastases (n=709)1
At a median follow-up of 72 months
1L patients were defined as those with de novo disease (no prior exposure to ET) and those with relapse >12 months from the end of (neo)adjuvant ET (late relapse).1
Study design: This MONALEESA pooled analysis included 1889 patients from across the MONALEESA trials, of which 59.5% (n=1124) had visceral metastases; of the 1229 patients receiving 1L therapy, 57.7% (n=709) had visceral metastases. These results are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.1
MONALEESA trial information
MONALEESA-2 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + letrozole (n=334) vs placebo + letrozole (n=334) in postmenopausal patients with HR+/HER2- mBC who received no prior therapy for advanced disease. OS was a secondary end point; PFS was the primary end point. At a median follow-up of 80 months, mOS was 63.9 months with KISQALI + letrozole (95% CI: 52.4-71.0) vs 51.4 months with placebo + letrozole (95% CI: 47.2-59.7); HR=0.765 (95% CI: 0.628-0.932); P=0.004.2,5,6
MONALEESA-3 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + fulvestrant (n=484) vs placebo + fulvestrant (n=242) in postmenopausal patients with HR+/HER2- mBC who received no or only 1 line of prior ET for advanced disease. OS was a secondary end point; PFS was the primary end point. In an exploratory analysis of a 1L subgroup of patients receiving KISQALI + fulvestrant (n=237) or placebo + fulvestrant (n=128), at a median follow-up of 71 months mOS was 67.6 months with KISQALI + fulvestrant (95% CI: 59.6-NR) vs 51.8 months with placebo + fulvestrant (95% CI: 40.4-61.2); HR=0.673 (95% CI: 0.504-0.899). At a median follow-up of 39 months, statistical significance was established for overall survival in the ITT population; HR=0.724 (95% CI: 0.568-0.924); P=0.00455. Results from the 71-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.3,5,7,8
MONALEESA-7 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + ET (NSAI or tamoxifen) + goserelin (n=335) vs placebo + ET (NSAI or tamoxifen) + goserelin (n=337) (ITT) in premenopausal patients with HR+/HER2- mBC who received no prior ET for advanced disease. KISQALI is not indicated for concomitant use with tamoxifen. Efficacy results are from a prespecified subgroup analysis of 495 patients who received KISQALI (n=248) or placebo (n=247) with an NSAI + goserelin and were not powered to show statistical significance. OS was a secondary end point; PFS was the primary end point. At a median follow-up of 54 months (exploratory analysis), mOS was 58.7 months with KISQALI + NSAI + goserelin (95% CI: 48.5-NR) vs 47.7 months with placebo + NSAI + goserelin (95% CI: 41.2-55.4); HR=0.798 (95% CI: 0.615-1.035). At a median follow-up of 35 months, statistical significance was established for overall survival in the ITT population; HR=0.71 (95% CI: 0.54-0.95); P=0.00973. Results from the 54-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.4,5,9-11
1L, first line; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; ITT, intent to treat; mBC, metastatic breast cancer; mOS, median overall survival; NR, not reached; NSAI, nonsteroidal aromatase inhibitor; OS, overall survival; PFS, progression-free survival.
MONALEESA-3 SUBGROUP
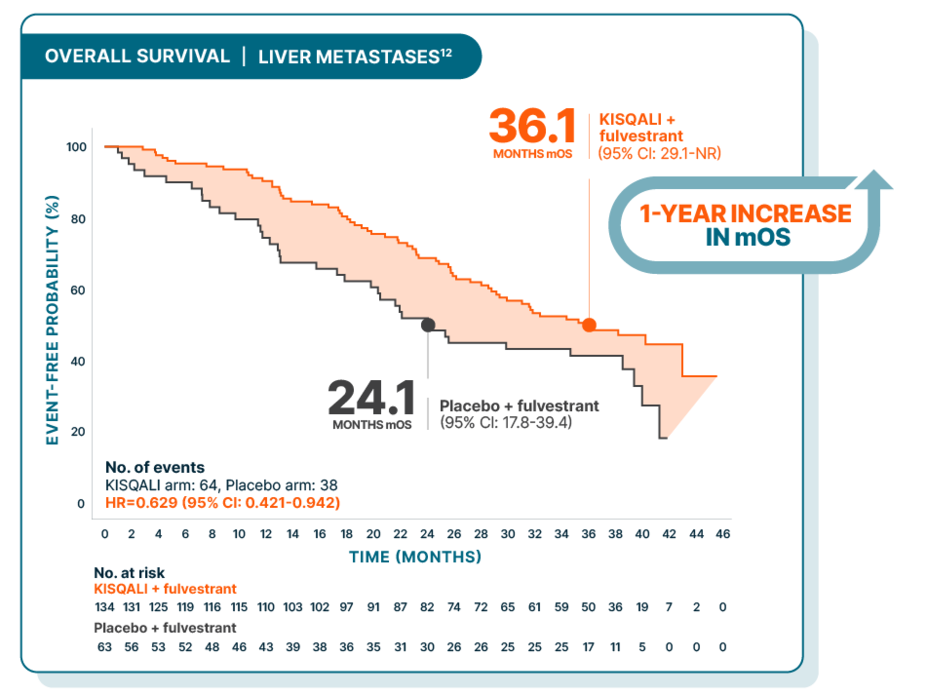
Overall survival improved in 1L/2L postmenopausal patients from the MONALEESA-3 trial, including those with liver metastases
At a median follow-up of 39 months
Hazard ratios are based on stratified Cox model.12
These results were exploratory in nature and should be interpreted with caution due to small n-sizes; as such, there was no prespecified statistical procedure controlling for type 1 error
In patients with visceral metastases: Median OS was 41.0 months with KISQALI + fulvestrant (n=293) (95% CI: 38.5-NR) vs 39.4 months with placebo + fulvestrant (n=147) (95% CI: 30.6-42.2); HR=0.804 (95% CI: 0.596-1.083)12
27% (197/726) of patients had liver metastases in MONALEESA-35,12
Safety profiles were consistent with the overall population: neutropenia (60.2%), nausea (46.6%), and diarrhea (34.6%) were the most common in the postmenopausal trial. The rates of grade 3/4 ALT/AST elevations in the KISQALI arm (13.5%) were similar to those seen in the overall safety population (12%)12
MONALEESA-3 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + fulvestrant (n=484) vs placebo + fulvestrant (n=242) in postmenopausal patients with HR+/HER2- mBC who received no or only 1 line of prior ET for advanced disease. OS was a secondary end point; PFS was the primary end point. In an exploratory analysis of a 1L subgroup of patients receiving KISQALI + fulvestrant (n=237) or placebo + fulvestrant (n=128), at a median follow-up of 71 months mOS was 67.6 months with KISQALI + fulvestrant (95% CI: 59.6-NR) vs 51.8 months with placebo + fulvestrant (95% CI: 40.4-61.2); HR=0.673 (95% CI: 0.504-0.899). At a median follow-up of 39 months, statistical significance was established for overall survival in the ITT population; HR=0.724 (95% CI: 0.568-0.924); P=0.00455. Results from the 71-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error.3,5,7,8
2L, second line; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
________________________________________________________________________________________________
RIGHT CHOICE TRIAL
KISQALI + AI + goserelin was studied vs combination chemotherapy in patients with aggressive disease
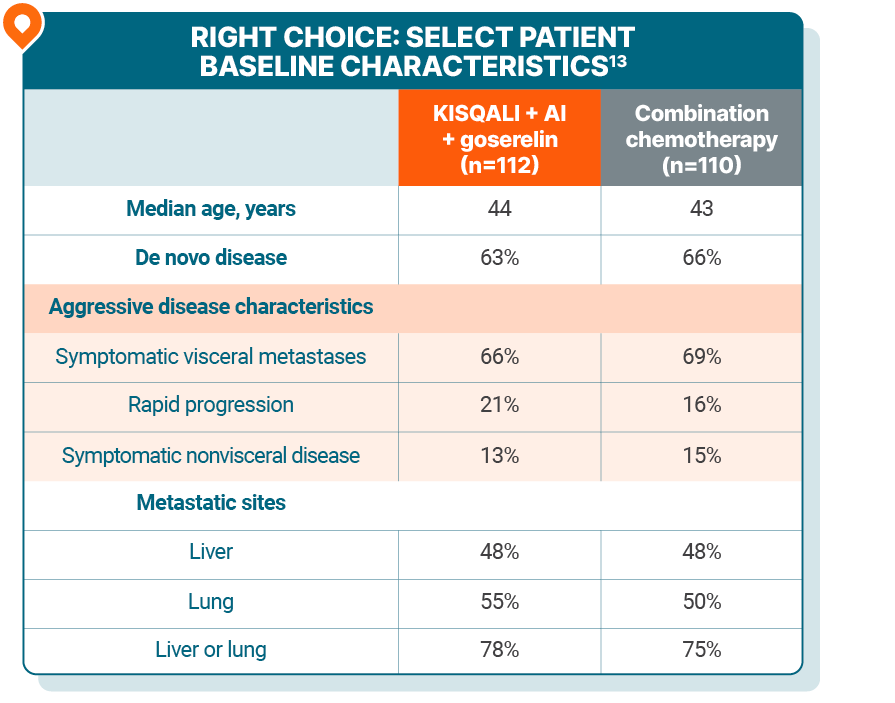
The RIGHT Choice trial: a phase II trial evaluating KISQALI + an AI + goserelin for 1L treatment of pre- or perimenopausal patients with HR+/HER2- mBC who have aggressive disease
Aggressive disease is characterized by rapidly growing cancer cells that are more likely to metastasize to other parts of the body. Aggressive breast cancer is generally associated with a higher risk of recurrence and poorer prognosis.
As determined by the investigators, 106 patients presented with visceral crisis and 116 patients presented without visceral crisis13
Visceral crisis, defined subjectively as severe organ dysfunction, as assessed by signs and symptoms, laboratory studies, and rapid progression of the disease, in patients with mBC often requires a treatment with rapid efficacy14
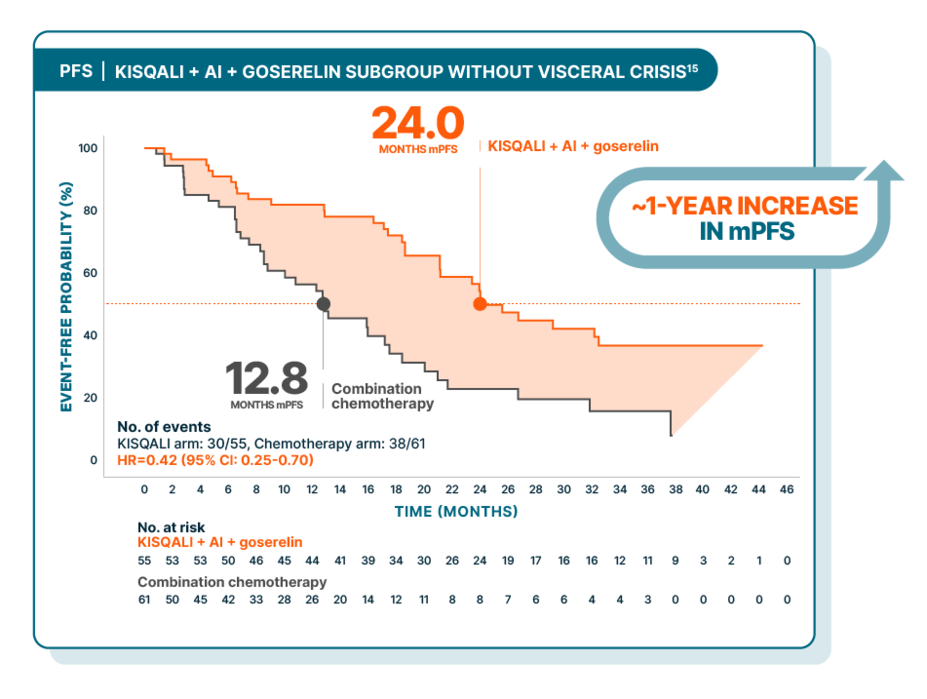
KISQALI + AI + goserelin vs combination chemotherapy in aggressive HR+/HER2- advanced breast cancer: subgroup analysis of patients without visceral crisis
Overall survival is being evaluated but is ongoing and currently not yet mature.16
KISQALI + AI + goserelin tumor response rates were consistent with combination chemotherapy13,15
KISQALI + AI + goserelin ORR: 60.0% (95% CI: 45.9-73.0)
Combination chemotherapy ORR: 62.3% (95% CI: 49.0-74.4)
Due to the nature of the study, results should be interpreted with caution. These results from a subgroup analysis of patients with aggressive disease who did not have visceral crisis are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.
RIGHT Choice was a randomized, phase II, open-label, multicenter study of KISQALI + AI + goserelin (n=112) vs combination chemotherapy (either docetaxel + capecitabine, paclitaxel + gemcitabine, or capecitabine + vinorelbine) (n=110) in pre- or perimenopausal patients with HR+/HER2- mBC who have aggressive disease. PFS was the primary end point. Efficacy results are from a subgroup analysis of patients with aggressive disease who did not have visceral crisis. In this subgroup, median PFS was 24.0 months with KISQALI + NSAI + goserelin (95% CI: 21.1-NE) vs 12.8 months with combination chemotherapy (95% CI: 8.5-17.5); HR=0.42 (95% CI: 0.25-0.70). Due to the nature of the study, results should be interpreted with caution. These results are exploratory and hypothesis-generating; as such, there was no statistical procedure controlling for type 1 error.13,16
AI, aromatase inhibitor; mPFS, median progression-free survival; NE, not estimable, ORR, overall response rate.