KISQALI—straightforward dosing
For your patients with HR+HER2- mBC,
Start with KISQALI 600 mg—the starting dose with proven outcomes
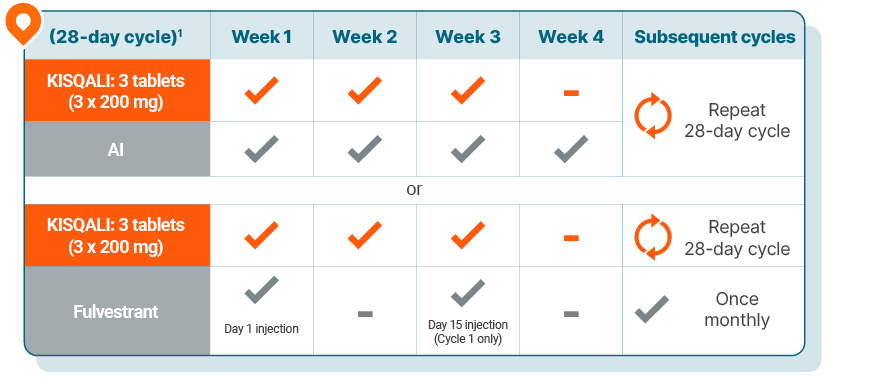
KISQALI is given as 600 mg (3 x 200-mg tablets) orally, once daily (3 weeks on, 1 week off) with either1:
An AI once daily (continuously); in men and premenopausal women, an LHRH agonist should also be administered according to current clinical practice guidelines; or
Fulvestrant 500 mg intramuscularly on Days 1, 15, and 29, and once monthly thereafter; in men and premenopausal women, an LHRH agonist should also be administered according to current clinical practice guidelines
Patients should continue treatment until disease progression or unacceptable toxicity
Please refer to the full Prescribing Information for fulvestrant
Please refer to the full Prescribing Information for the recommended dose of the chosen AI
KISQALI can be taken with or without food
Store refrigerated at 2°C to 8°C (36°F to 46°F). Excursions permitted between 2°C and 15°C (36°F and 59°F)
After dispensing, patients may store at room temperature at 20°C to 25°C (68°F to 77°F) for up to 2 months
Store tablets in the original blister pack
Starting dose modifications for hepatic and severe renal impairment1
The recommended starting dose is 400 mg once daily for patients with moderate or severe (Child-Pugh class B or C) hepatic impairment
The recommended starting dose is 200 mg once daily for patients with severe renal impairment
Expert perspective on dosing and patient adherence in HR+/HER2- mBC
Dr Nick McAndrew shares his perspective on simple dose reductions with KISQALI and how to improve adherence in patients with HR+/HER2- mBC.