KISQALI—straightforward assessments and less monitoring over time
Complete most of the scheduled assessments for KISQALI within the first 2 months of therapy—with none beyond Cycle 6
Plan ahead—work with your patients to schedule these assessments as soon as possible to help them start and stay on therapy
Monitoring requirements based on a 28-day treatment cycle.
Additional monitoring may be required as clinically indicated.
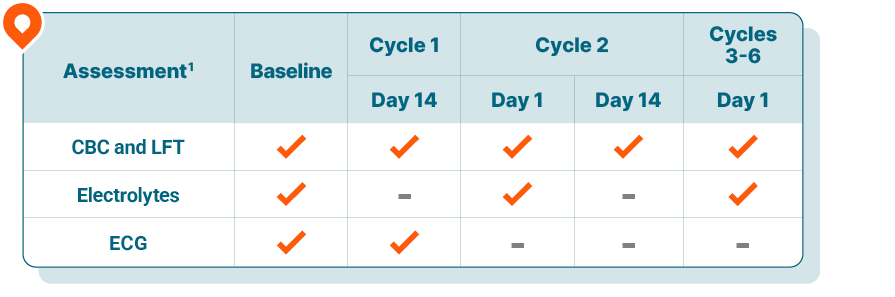
Routine monitoring for lab abnormalities1
Blood tests are performed at baseline, on Day 14 of Cycle 1, on Days 1 and 14 of Cycle 2, on Day 1 of Cycles 3 through 6, and as clinically indicated
For LFTs, if grade ≥2 abnormalities are noted, more frequent monitoring is recommended
Correct any electrolyte abnormalities prior to treatment
2 required ECG assessments completed within the first 2 weeks of treatment1
ECGs are performed at baseline, on Day 14 of Cycle 1, and as clinically indicated
KISQALI should only be initiated in patients with QTcF <450 ms
In case of QTcF prolongation during therapy, more frequent assessments are recommended
The majority of adverse reactions with KISQALI were manageable and reversible
Review the dose adjustment guidance and safety information for KISQALI
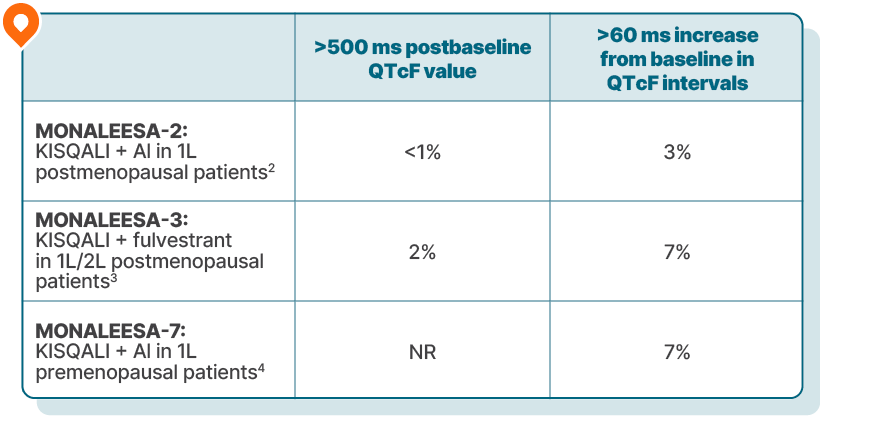
Low incidence of QT prolongation across KISQALI trials
In a pooled analysis across 3 phase III trials of 1054 premenopausal and postmenopausal patients treated with KISQALI + an AI or fulvestrant1:
There were no reported cases of torsades de pointes.1
Most cases of QT prolongation in the pooled analysis were moderate in nature
A brochure addressing perceptions about QT prolongation with KISQALI
ECG device monitoring program
A flexible program designed to meet the needs of you and your patients’ treatment plans.
The KISQALI ECG Device Monitoring Program can provide you or your patients with a KardiaMobile 6L ECG device so ECG assessments can be completed in seconds in your office or at home.
In-Office option for eligible practices to receive a fast and simple-to-use ECG monitoring device that will allow them to easily monitor their patients taking or being evaluated for KISQALI
Direct-to-Patient option designed so patients can complete their ECG without a trip to the office
There is no direct cost to you or your patients for participating in this program
Actual ECG reading takes seconds and fits into any office workflow. QTcF results from the ECG device are available instantaneously, with the option for a board-certified cardiologist overread
Limitations apply. KISQALI ECG Device Monitoring Program is only permitted to be used for monitoring or evaluating a patient for the current or potential administration of ribociclib. The equipment or services are not permitted to be used for any purpose outside of the scope of the program. You must not bill any entity or person for any equipment or services relating to the provision or interpretation of the ECG. In the event that you fail to abide by the rules of the KISQALI ECG Device Monitoring Program, your participation in the program may be terminated or modified at any time without prior notice, and you may be subject to additional remedies. Additional terms and conditions apply.
Sunshine Act costs may apply.
For additional information, please contact your Novartis Oncology Specialist.
Learn more about the AliveCor® KardiaMobile 6L ECG device and how to use it
In-Office ECG monitoring with AliveCor® KardiaMobile 6L