More life for living
MONALEESA-3: KISQALI + fulvestrant in postmenopausal patients, data from the 1L subgroup
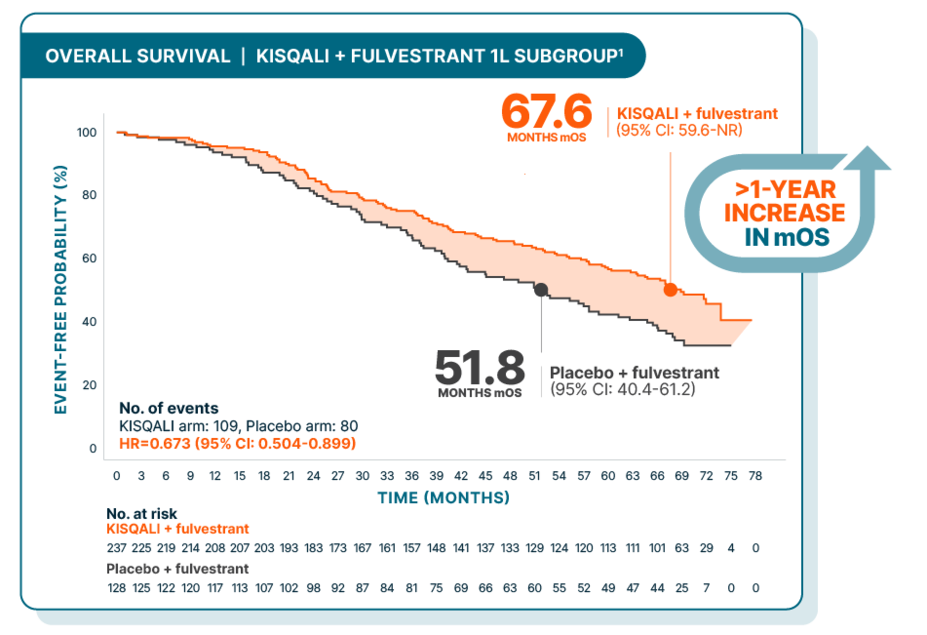
OVERALL SURVIVAL
Over 5.5 years median overall survival for 1L postmenopausal patients with fulvestrant
At a median follow-up of 71 months
Hazard ratio is based on unstratified Cox model.2
Results from the 71-month analysis were not prespecified and were observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
Statistically significant improvement in overall survival in the 1L/2L ITT population3,4:
At a median follow-up of 39 months, mOS with KISQALI + fulvestrant was not reached (95% CI: 42.5-NR) vs 40.0 months with placebo + fulvestrant (95% CI: 37.0-NR); P=0.00455; HR=0.724 (95% CI: 0.568-0.924)
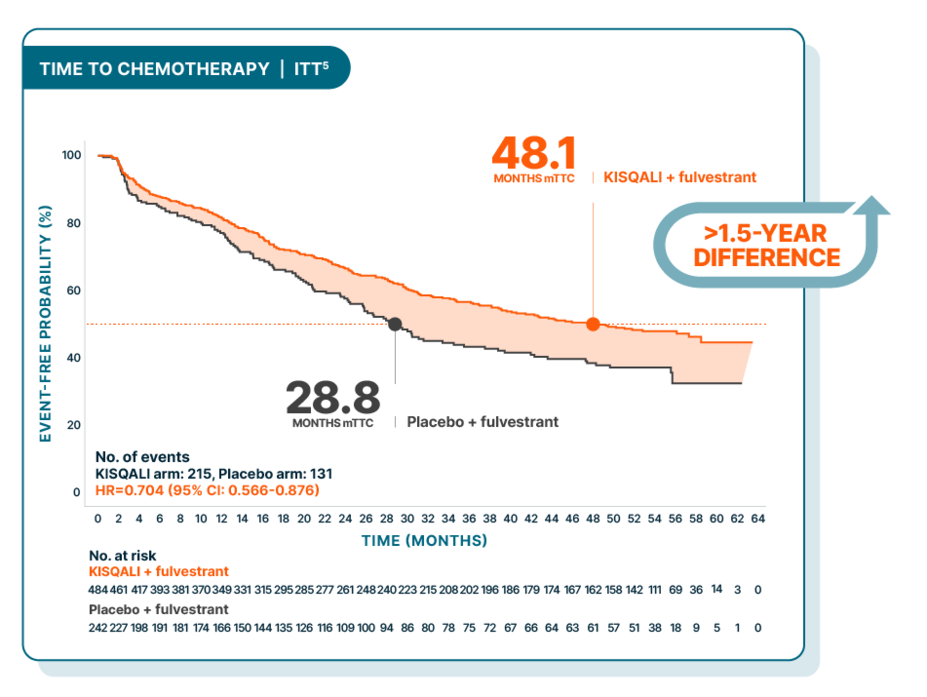
TIME TO CHEMOTHERAPY
Median time to chemotherapy delayed 4 years
At a median follow-up of 56 months
Hazard ratio is based on stratified Cox model.3
Time to chemotherapy was an exploratory end point and was defined as the time from randomization to the beginning of the first chemotherapy after discontinuing study treatment3
There was no prespecified statistical procedure controlling for type 1 error
Study design: MONALEESA-3 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + fulvestrant (n=484) vs placebo + fulvestrant (n=242) for the treatment of postmenopausal patients with HR+/HER2- mBC who received no or only 1 line of prior ET for advanced disease. OS was a secondary end point; PFS was the primary end point.4,6