More life for living
KISQALI—the only CDK4/6 inhibitor to achieve statistically significant overall survival in first line in combination with an AI
MONALEESA-2: KISQALI + AI in 1L postmenopausal patients
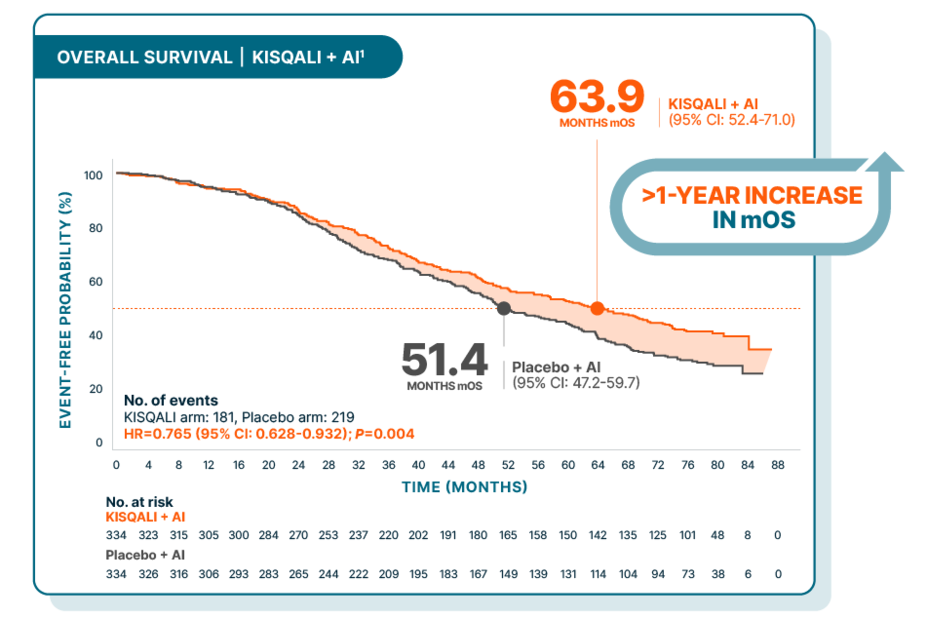
OVERALL SURVIVAL
Over 5 years median overall survival for 1L postmenopausal patients with an AI
At a median follow-up of 80 months
Hazard ratio is based on stratified Cox model.2
OS benefit with KISQALI increased over time
At 6 years, the survival rate of patients receiving KISQALI + letrozole was 44% vs 32% with placebo + letrozole2
1L, first line; AI, aromatase inhibitor; CDK, cyclin-dependent kinase; HR, hazard ratio; mOS, median overall survival; OS, overall survival.
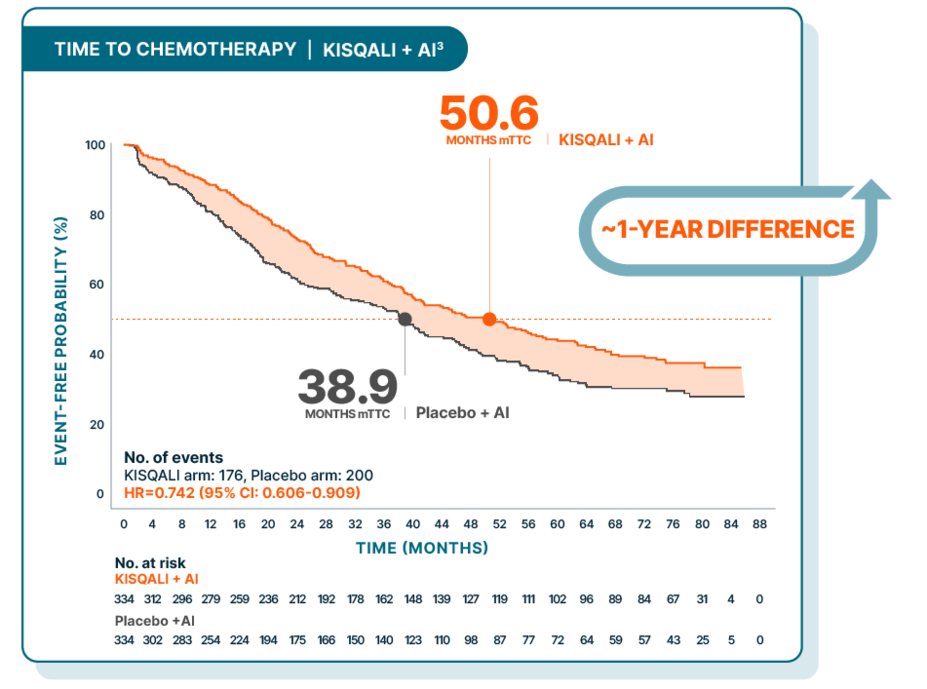
TIME TO CHEMOTHERAPY
Median time to chemotherapy delayed over 4 years
At a median follow-up of 80 months
Time to chemotherapy was evaluated in a post hoc exploratory analysis and was defined as the time from randomization to the beginning of the first chemotherapy after discontinuing study treatment. There was no prespecified statistical procedure controlling for type 1 error
MONALEESA-2 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + letrozole (n=334) vs placebo + letrozole (n=334) in postmenopausal patients with HR+/HER2- mBC who received no prior therapy for advanced disease. OS was a secondary end point; PFS was the primary end point.1,2,5
HER2-, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; mBC, metastatic breast cancer; mTTC, median time to chemotherapy; PFS, progression-free survival.