MONALEESA-7 safety profile
KISQALI + AI in 1L premenopausal patients
Majority of adverse reactions were manageable and reversible1-3
These safety data are based on 495 patients who received KISQALI + NSAI + goserelin or placebo + NSAI + goserelin in MONALEESA-7:
Dose reductions due to ARs: 33% with KISQALI + NSAI + goserelin4
Permanent discontinuations: 3% with KISQALI + NSAI + goserelin4
Patients may require dose interruption, reduction, or discontinuation for ARs. Monitoring should include pulmonary symptoms, ECGs, serum electrolytes, LFTs, and CBCs. See Warnings and Precautions for risk of ILD/pneumonitis, SCARs, QT prolongation, hepatotoxicity, neutropenia, and embryo-fetal toxicity4
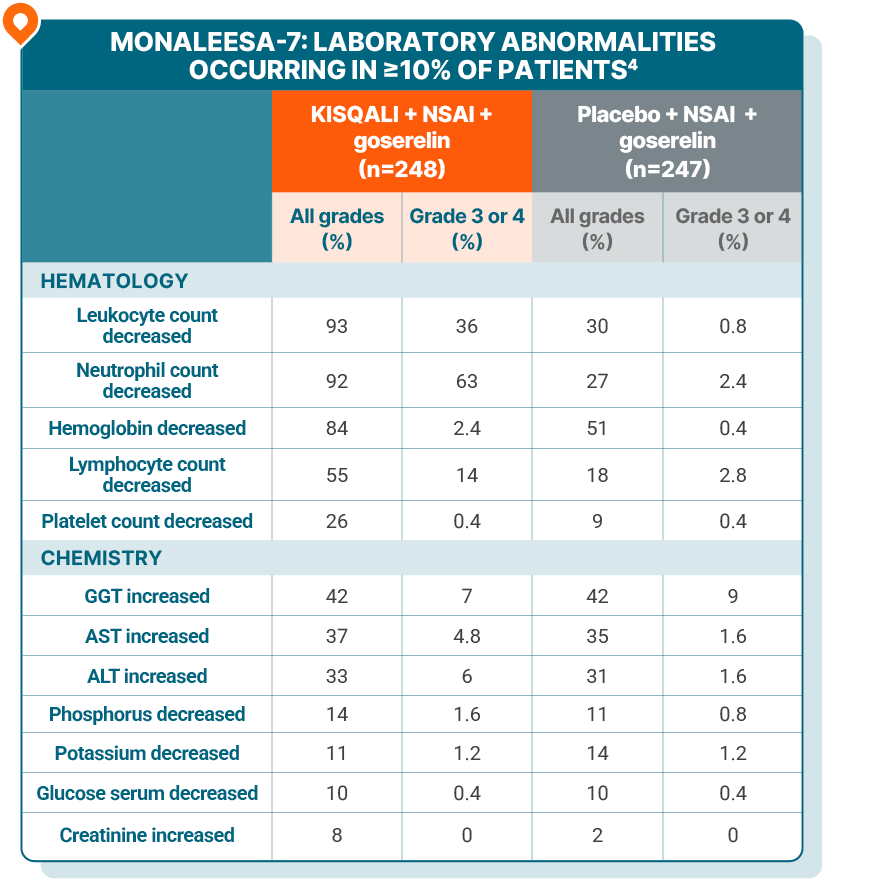
The most common ARs (≥20% on the KISQALI arm and ≥2% higher than placebo), including laboratory abnormalities, were decrease in leukocytes, decrease in neutrophils, decrease in hemoglobin, decrease in lymphocytes, increase in gamma-glutamyl transferase, increase in AST, infections, arthralgia, increase in ALT, nausea, decrease in platelets, and alopecia4
ARs in patients with visceral metastases receiving KISQALI were consistent with ARs in those without visceral metastases5
Grading according to CTCAE version 4.03.
*Infections included urinary and respiratory tract infections, gastroenteritis, and sepsis (<1%).
†Only includes grade 3 ARs.
Scheduled blood tests and 2 upfront ECGs help to ensure your patients start KISQALI with confidence
Review the assessments schedule and the incidence of QT prolongation across clinical trials
The majority of adverse reactions with KISQALI were manageable and reversible
Review dose adjustment guidance