Patient-reported health-related quality of life with KISQALI + ET and ET alone in HR+/HER2- mBC
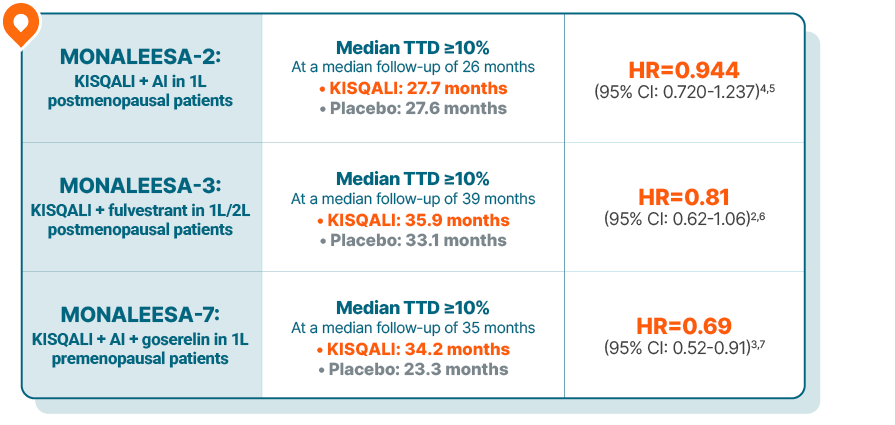
Time to deterioration (TTD) ≥10% across the MONALEESA trials1-3
HRQOL was assessed using the EORTC QLQ-C30 questionnaire—a validated tool used worldwide to assess quality of life in patients with cancer.1-3,8,9
HRQOL was a secondary end point measured by patient-reported outcomes and was assessed at baseline and every 8 to 12 weeks throughout treatment
TTD was defined as a decline of at least 10% of the global health status/QOL scale score or death due to any cause
There was no prespecified statistical procedure controlling for type 1 error
The EORTC QLQ-C30 is not all inclusive and does not include adequate assessment of all expected treatment-related symptoms. TTD may be confounded by events not related to disease/treatment
MONALEESA-2 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + letrozole (n=334) vs placebo + letrozole (n=334) in postmenopausal patients with HR+/HER2- mBC who received no prior therapy for advanced disease. OS was a secondary end point; PFS was the primary end point.10-12
MONALEESA-3 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + fulvestrant (n=484) vs placebo + fulvestrant (n=242) in postmenopausal patients with HR+/HER2- mBC who received no or only 1 line of prior ET for advanced disease. OS was a secondary end point; PFS was the primary end point.10,13
MONALEESA-7 was a randomized, double-blind, placebo-controlled, phase III study of KISQALI + ET (NSAI or tamoxifen) + goserelin (n=335) vs placebo + ET (NSAI or tamoxifen) + goserelin (n=337) (ITT) in premenopausal patients with HR+/HER2- mBC who received no prior ET for advanced disease. KISQALI is not indicated for concomitant use with tamoxifen. Efficacy results are from a prespecified subgroup analysis of 495 patients who received KISQALI (n=248) or placebo (n=247) with an NSAI + goserelin and were not powered to show statistical significance. OS was a secondary end point; PFS was the primary end point.10,14
1L, first line; 2L, second line; AI, aromatase inhibitor; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HRQOL, health-related quality of life; HR+, hormone receptor-positive; ITT, intent to treat; mBC, metastatic breast cancer; NSAI, nonsteroidal aromatase inhibitor; OS, overall survival; PFS, progression-free survival; QOL, quality of life; TTD, time to deterioration.